Say Goodbye to LID.
Plasmalogens and Parkinson’s disease
Reduced levels of plasmalogens have been reported in the brain and circulation of individuals with Parkinson’s disease (1, 2). Plasmalogen levels in neuronal membranes are critical for their function, with reduced levels leading to impaired vesicular fusion (3, 4) and neurotransmitter protein binding (5, 6, 7). In addition, plasmalogen levels are associated with maintaining normal alpha-synuclein interaction with the presynaptic membrane (8, 9), reducing the propensity for synuclein fibrils (10, 11) which are central to the pathology of Parkinson’s disease.
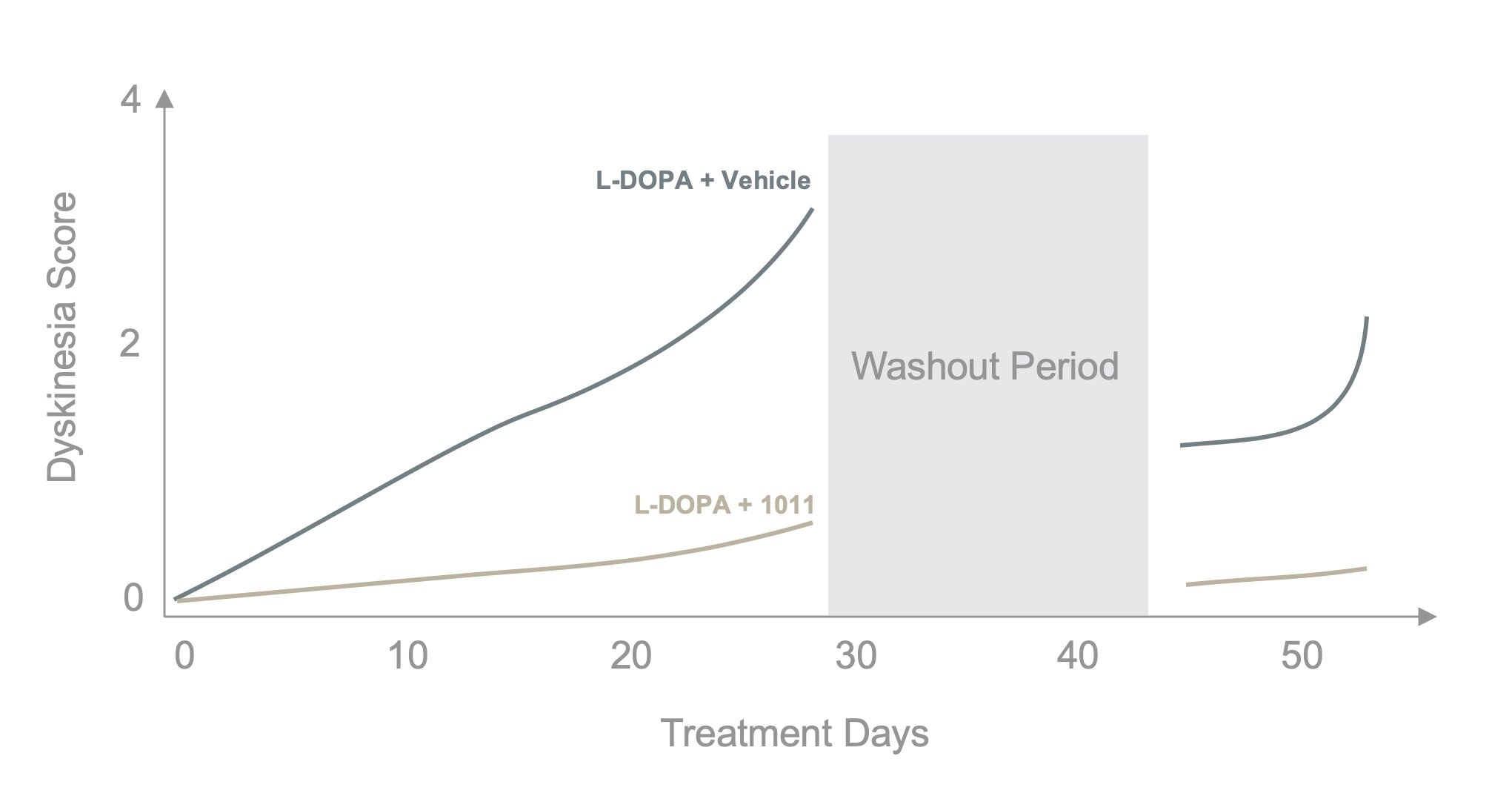
Protection Against Levodopa-Induced Dyskinesia
Levodopa (L-DOPA) is currently the most commonly prescribed and effective symptomatic treatment for Parkinson’s disease. Despite its widespread use and initial efficacy, the vast majority of patients develop L-DOPA-induced dyskinesias (LID), involuntary muscle movements or tremors, after 5-10 years of treatment, limiting the utility of L-DOPA. Severity of symptoms is patient dependent, but in many cases LID is extremely challenging to manage and becomes disabling, greatly reducing quality of life.
GraySpace Therapeutics has been investigating the therapeutic utility of plasmalogens in the treatment of LID. When PPI-1011 is administered concurrently with Levodopa, dyskinesias do not develop (see graph above), even following washout (12). Further, established LID can also be reduced with PPI-1011 treatment (13).
PPI-1011 offers a novel approach to significantly improve the therapeutic window of L-DOPA, which could radically improve the disease management of Parkinson’s disease.
Neuroprotection
The 1-methyl-4-phenyl-1,2,3,6-tertrahydropyridine (MPTP) neurotoxic model of Parkinson’s disease is the most commonly used animal model system, as the effects on the brain closely mirror those found in human Parkinson’s disease patients. MPTP-treatment also reduces plasmalogen levels.
At GraySpace, we have been investigating the neuroprotective ability of plasmalogen augmentation and have shown that PPI-1011 treatment not only protects against the reduction in plasmalogen levels following MPTP exposure, it also protects against the MPTP-induced reduction in neurotransmitter levels (serotonin and dopamine). Additionally, PPI-1011 treatment maintains normal binding efficiency of two critical vesicularly located neurotransmitter transporters (VMAT2 and DAT) (14).
PPI-1011’s ability to rescue the Parkinsonian phenotype in the striatum supports a disease-modifying effect of treatment and represents a new way of protecting against the neurodegeneration central to the progression of Parkinson’s disease.
Gut Inflammation
Parkinson’s disease is typically thought of as a disease of the central nervous system, however increasing data shows that changes in the gut, including increased inflammation and impaired dopaminergic function, are among the earliest symptoms of the disease. The MPTP-model used above also recapitulates this phenotype and was used to evaluate the systemic effects of plasmalogen augmentation.
PPI-1011 is also neuroprotective in the gastrointestinal tract, and protects against the loss of tyrosine hydroxylase production (which is central for normal dopaminergic signaling within the gut). Treatment also reduces inflammation in the gut by preventing the infiltration of macrophages typically seen in response to MPTP-administration (15). These effects are independent of whether treatment is given before or after the administration of MPTP.
PPI-1011 therefore represents a potentially new opportunity for treating both the systemic and central nervous system manifestations of Parkinson’s disease, which would be a significant advancement in the therapeutic management of patients.

“The fact that this is the first drug that appears to completely prevent the establishment of LID could be a game-changer for Parkinson’s patients.”
— Barry Markowsky, CEO